Technology
Our integrated liver cell manufacturing process produces billions of top quality human hepatocytes.
Overview
In 2008 Japanese researcher Shinya Yamanaka discovered how to take ordinary skin cells, turn back their genetic clock, and transform them into the equivalent of embryonic stem cells. Since stem cells can grow into virtually any type of cell in the body, the breakthrough offered hope of treatment for a wide range of diseases. For the past decade scientists worldwide have been devising protocols to unlock the potential of iPS-derived stem cells.
Von Baer Wolff focused on developing an integrated protocol to fabricate human hepatocytes, the primary cells of the liver. A plentiful supply of these cells will propel science and industry, enabling new drugs and medical therapies. Our technology delivers consistent, high quality iPS-derived human hepatocytes in the quantities needed to be commercially viable.
How It Works
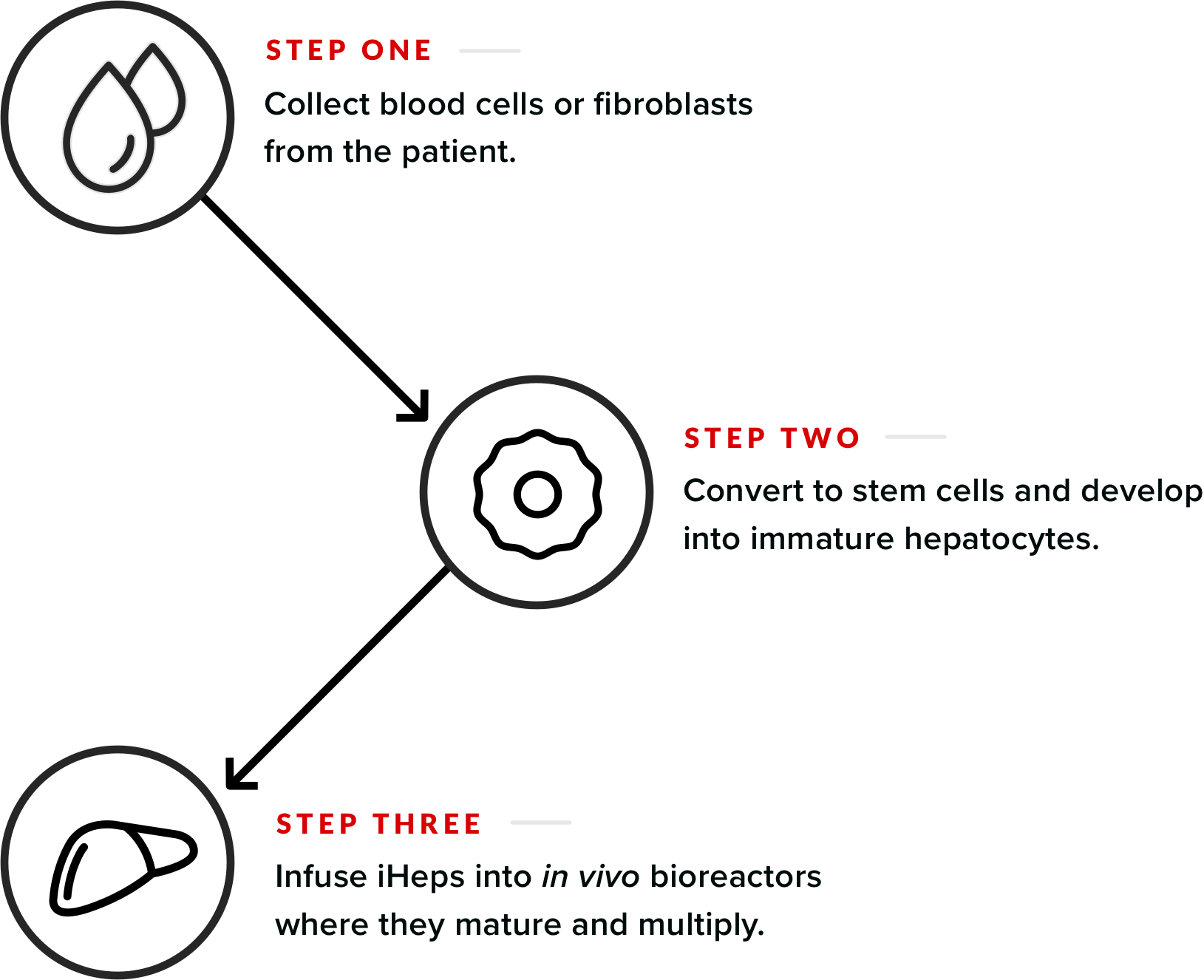
Our three-step proprietary approach begins with the collection of blood cells or fibroblasts from a person’s skin. The blood cells or fibroblasts are converted to stem cells and then developed into immature hepatocytes called iHeps. The iHeps are infused into in vivo bioreactors—transgenic animals—where they mature and multiply. Each bioreactor produces between half a billion to a billion primary human hepatocytes with consistent high-metabolic functionality.
By inserting an extra step in this process we can address a range of metabolic liver diseases caused by single-gene mutations. Blood cells or fibroblasts are collected from the affected patient and differentiated into iHeps. Then, using CRISPR/Cas9 gene-editing technology, we correct the genetic defect. The autologous gene-edited iHeps are infused into in vivo bioreactors to mature and multiple.
When the healthy, gene-corrected hepatocytes are infused into the patient’s liver, they function and regenerate normally. Because the corrected hepatocytes are patient specific, they do not require immune suppression. Gene-edited hepatocyte transplant effectively offers a curative therapy for congenital liver diseases like alpha-1 antitrypsin deficiency, hereditary hemochromatosis, phenylketonuria, Wilson's disease and many others.
Our innovative technology delivers:
Consistency
High-quality, fully functional human hepatocytes.
Reliability
A commercial hepatocyte supply pipeline.

Affordability
Competitive prices for a far superior product.
Opportunities
Multiple ways to collaborate.